- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
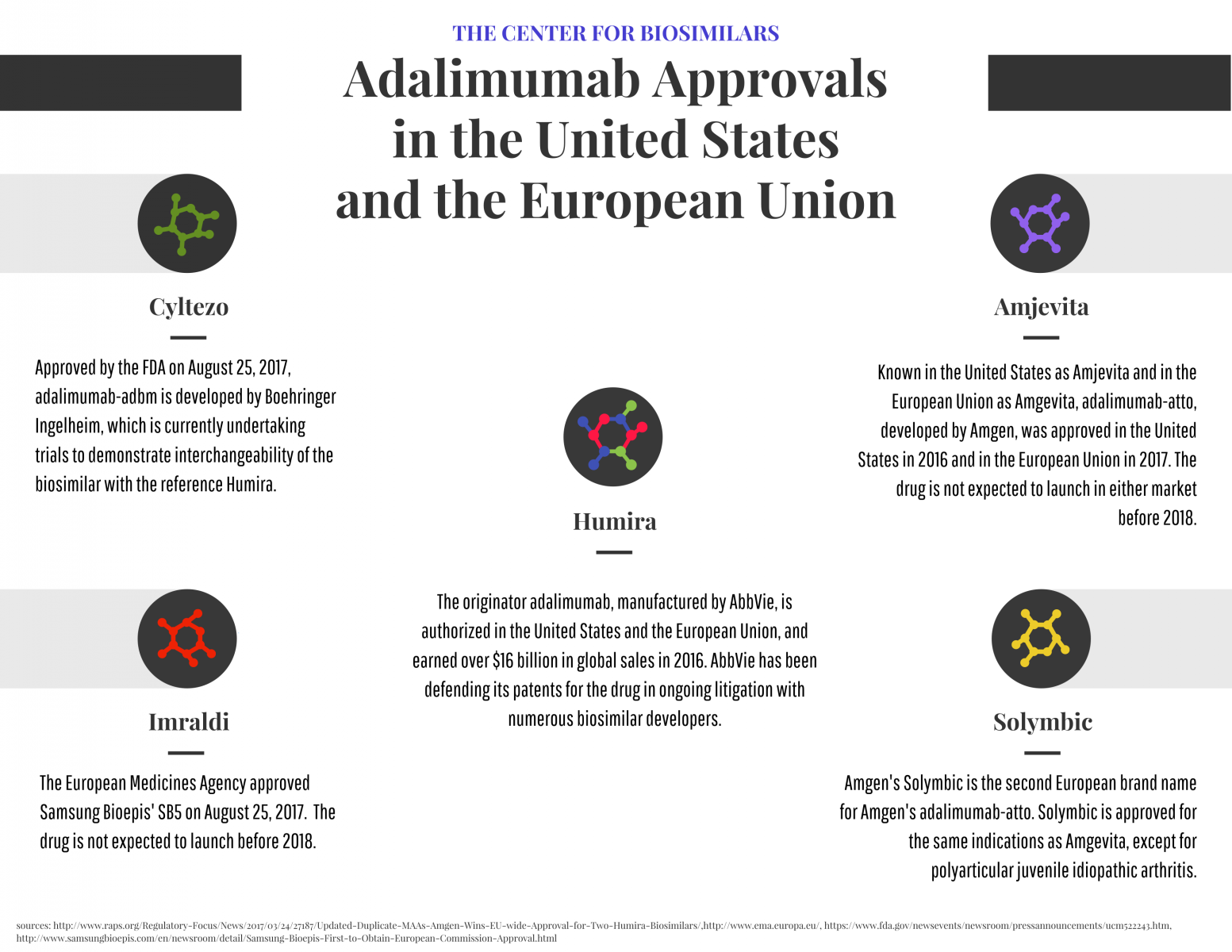
Infographic: Adalimumab Approvals in the United States and European Union
Competition for the $16 billion market for Humira is heating up with new approvals for adalimumab biosimilars in the United States and European Union.
Competition for the $16 billion market for Humira is heating up with new approvals for adalimumab biosimilars in the United States and European Union.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
Related Content