- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Biosimilars Poll: What is Your Opinion on Clinical Efficacy Trials?
There are diverging opinions on whether comparative efficacy trials add much of value to the evidence for biosimilar approval in the United States.
Take the Biosimilars Poll!

There are diverging opinions on whether comparative efficacy trials add much of value to the evidence for biosimilar approval in the United States.
Which of the following best describes your opinion?
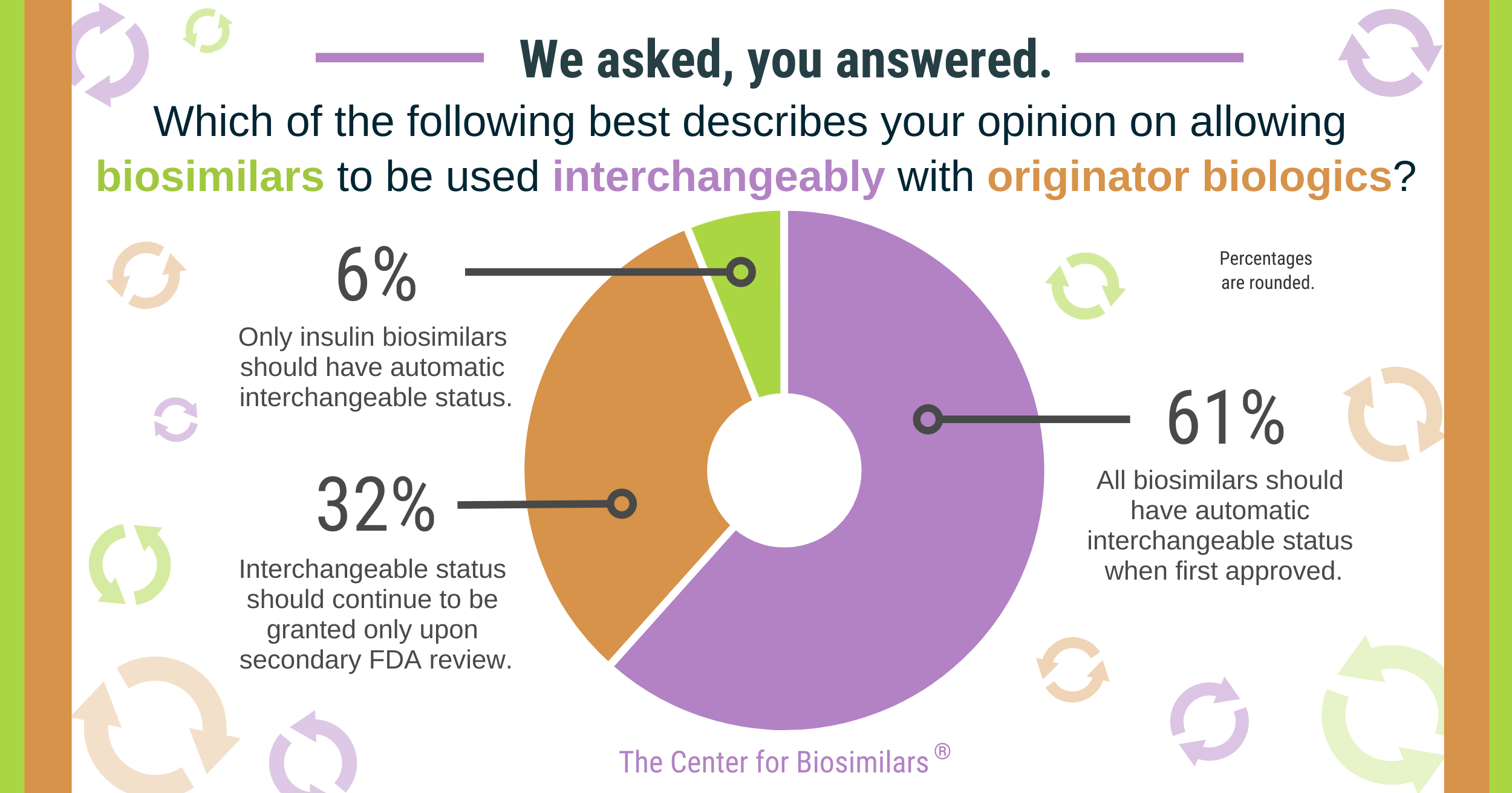
Check out the results from our last poll:
Click to enlarge
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
Related Content
