- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
FDA Updates Biosimilar Regulatory Research Program With Roadmap
The FDA published its research roadmap as part of the Biosimilars User Fee Act III regulatory research pilot program. The roadmap will allow stakeholders to see how the program will help the FDA enhance regulatory decision-making surrounding biosimilar development.
The FDA published its research roadmap as part of the Biosimilars User Fee Act (BsUFA) III regulatory research pilot program. The roadmap will allow stakeholders to see how the program will help the FDA enhance regulatory decision-making surrounding biosimilar development.
“FDA anticipates that the biosimilar and interchangeable landscape will continue to evolve. As such, both regulatory experience and policy development may inform and change the knowledge gaps for the research pilot program as BsUFA III progresses,” the agency wrote.
BsUFA allows for the FDA to utilize user fee revenue for the review of biosimilar product submissions. The first authorization of the act took place from 2013 to 2017 and enabled the development of the initial infrastructure needed to support the program for reviewing submissions. During the second installment (BsUFA II) from 2018 through 2022, the FDA focused on “effective scientific coordination and review consistency through review, procedural, and meeting performance enhancements.”
BsUFA III (2023-2027) builds on the first 2 authorizations by having the FDA take extra steps to ensure efficient governance and operations across the biosimilar product review programs. As part of the new authorization, the FDA penned a commitment letter establishing the regulatory science research program as a means of identifying knowledge gaps and researching ways to advance biosimilar development. The program features 2 demonstration projects:
- Advancing the development of interchangeable biosimilars
- Improving the efficiency of biosimilar project development
The first project will focus on generating information and methodologies to meet the safety standards for establishing interchangeability, especially for predicting immunogenicity and assessing differences in product presentations and container closure systems. The second project will work to enhance efforts to streamline the biosimilar development process and highlight methodology development to predict immunogenicity and conduct analytical and pharmacological assessments.
The FDA identified 2 areas of improvement that it deemed “essential” for achieving its projects, including increasing the accuracy and capability of analytical and CMC (chemistry, manufacturing, and controls) characterizations as well as developing alternatives or reducing the size of human clinical studies. The aim is to rework the regulatory process to focus on more comparative analytical assessments and less on the clinical pharmacology assessments and comparative clinical studies. The FDA will have 10 research priorities that will result in regulatory impact (Figure).
Click to enlarge.
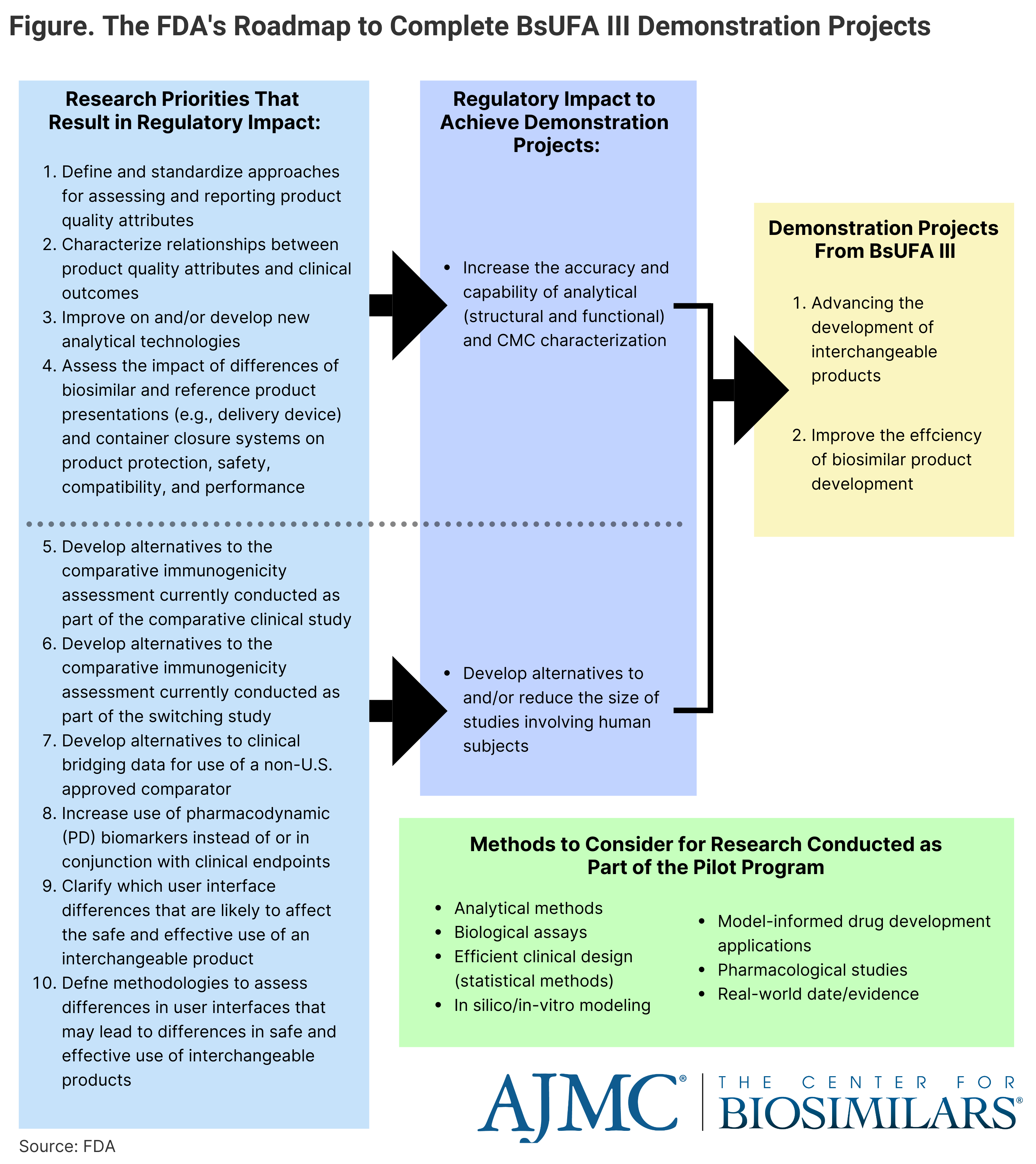
Developing several methodologies will be prioritized to ensure the FDA meets the goals outlined in BsUFA III. These methodologies may include analytical methods, biological functional assays, efficient clinical study design, in silico/ in vitro modeling, model-informed drug development applications, pharmacological studies, and real-world evidence.
As outlined in the commitment letter, the FDA will provide interim progress reports and workshops (on or before October 31, 2025) as well as a final summary report (on or before September 30, 2027) and a comprehensive strategy document that will outline specific actions the FDA will take to facilitate the development of biosimilars and interchangeable products (within 12 months post completion of the demonstration projects).
“FDA welcomes all stakeholder input on the regulatory research pilot program and its ability to enhance regulatory decision-making and facilitate science-based recommendations in areas foundational to biosimilar development,” the authors noted.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
