- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Genentech, Roche Recall Ophthalmology Biobetter Susvimo
Genentech and Roche have voluntarily recalled their ophthalmology biobetter, Susvimo, an ocular implant for ranibizumab injection, in the United States for a possible leakage issue.
Genentech, a member of the Roche group, announced a voluntariy recall of Susvimo, an ocular implant for ranibizumab injection that is a biobetter for Lucentis (ranibizumab), in the United States, according to a report from Ophthalmology Times.
Susvimo is an ocular implant of ranibizumab injection that is a biobetter of Lucentis (originator ranibizumab)

“The voluntary recall is based on recent testing of our commercial supply in which Susvimo implants were exposed to repeated puncturing with a needle…The results showed that some implants did not perform to our standards…. We deeply regret any disruption that this may cause to people with wet AMD and the retinal community,” Genentech explained in a statement.
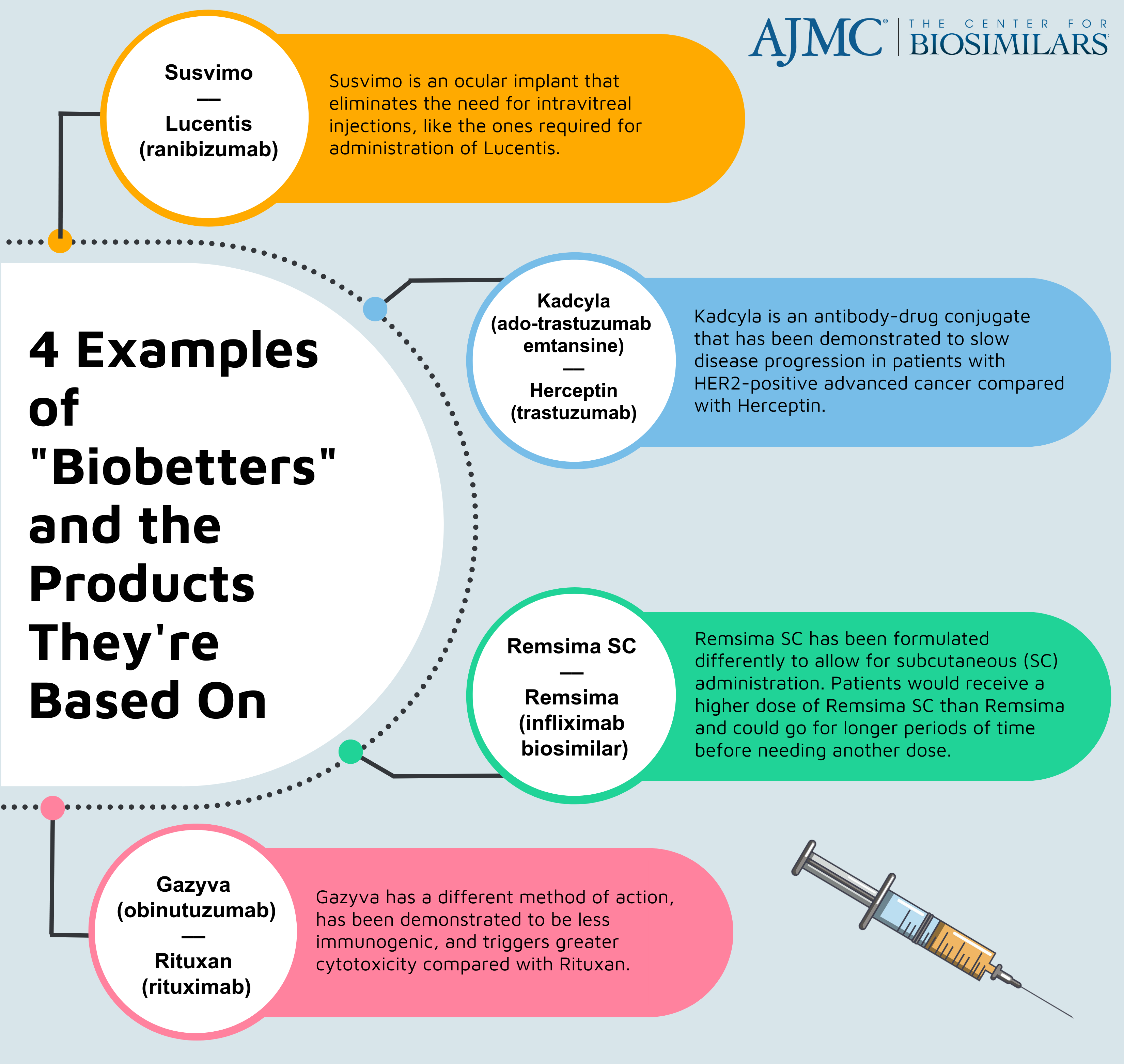
Biobetters are a class of follow-on biologic products that are intentionally altered to improve clinical effects, allow for more time in between doses, or enhance tolerability.
Susvimo is used as an insertion tool for the treatment of people with neovascular age-related macular degeneration (wet AMD). Susvimo offers benefits over Lucentis in that Susvimo contains a reservoir of ranibizumab that can last for 6 months, enabling patients to require less trips to the doctor’s office over the course of a year, thereby increasing medication adherence and making it easier for patients to manage wet AMD. By contrast, regular injections of Lucentis or a ranibizumab biosimilar are required as often as once a month.
The product was approved by the FDA in October 2021. Following the approval, 2 ranibizumab biosimilars (Byooviz and Cimerli) referencing the originator (Lucentis) have enter the market, including one with an interchangeability designation (Cimerli). The approval of Susvimo raised concerns for biosimilar manufacturers, as many patients may be persuaded to switch from the originator to the ocular implant before patient have has the opportunity to try a biosimilar that is priced lower than both products.
4 Examples of Biobetters and the Products They're Based On
According to Roche’s most recent earnings report, Susvimo accumulated about $3 million in revenue over the last quarter. Additionally, Lucentis generated about $800 million in quarterly revenues, a 25% drop from the previous quarter, which the company attributed to the market introduction of Byooviz (ranibizumab-runa) at the beginning of the third quarter of 2022.
Genentech said that it has notified the FDA and is working with the agency on the recall process. It also claimed that it has taken “immediate steps to inform other health authorities, healthcare professionals, clinical trial investigators, and patient organizations” and it will offer support and guidance to help maintain clinical outcomes for patients. The company recommended that health care providers discuss the continuation of treatment with their patients who are treated with Susvimo and it said that it will be closely monitoring patients in line with FDA-approved label and clinical trial protocols.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
