- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
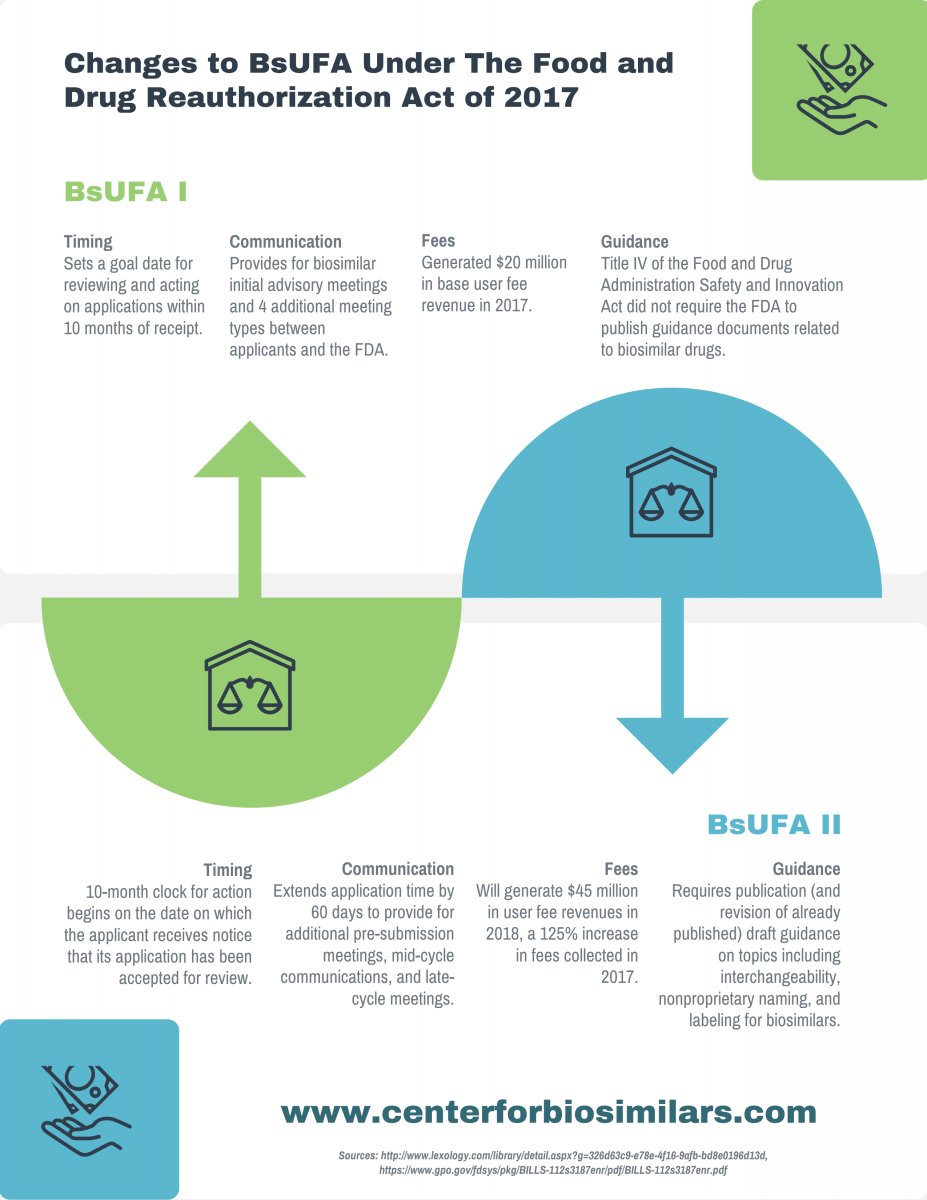
Infographic: Changes to BsUFA Under FDARA
The Food and Drug Reauthorization Act of 2017, recently passed by the House, reauthorizes the Biosimilar User Fee Act (BsUFA). Learn about changes to the BsUFA in its new iteration, BsUFA II.
The Food and Drug Reauthorization Act of 2017, recently passed by the House, reauthorizes the Biosimilar User Fee Act (BsUFA). Learn more about changes to the BsUFA in its new iteration, BsUFA II.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
Related Content