- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Nova Scotia Becomes Fifth Canadian Province to Implement a Biosimilar Switching Program
The province is the latest jurisdiction to adopt a program that will require patients to switch away from expensive reference biologics to select biosimilars for certain conditions.
Nova Scotia has become the fifth province and sixth jurisdiction in Canada to implement a switching policy that allows for reference products to be automatically substituted with biosimilars.
“The evidence is clear that biosimilars are a safe and effective form of drug therapy for many patients….If we’re able to provide the same high-quality treatment to our patients for lower cost, it’s our duty to do so,” said Michelle Thompson, minister of Health and Wellness, in a statement. "The cost savings from this change will be reinvested in healthcare, meaning even more people will get the care and treatment they deserve."
The province follows in the footsteps of British Columbia, New Brunswick, Alberta, Quebec, and the Northwest Territories, the last of which announced the implementation of a similar policy in December 2021.
Quebec announced its policy in May 2021, New Brunswick in April 2021, and Alberta in December 2019. British Columbia was the first to announce such a program in May 2019.
“As half of Canada’s provinces have recognized, implementing biosimilar switching policies is key to ensuring the sustainability of drug programs and making the most effective use of limited public funds….I encourage other provinces to follow the lead of Nova Scotia and other jurisdictions in moving forward with biosimilar switching policies without delay,” said Jim Keon, president of Biosimilars Canada, a national association representing the biosimilar medicines industry in Canada, in a statement with the organization.
Details of the Program
About 5100 patients who have health coverage with the Nova Scotia Pharmacare Programs treated with certain expensive reference biologic drugs to treat arthritis, diabetes, inflammatory bowel disease (IBD), or psoriasis will be switch to a biosimilar version of the medicine within the next 12 months, specifically by February 3, 2023.
The strategy is expected to help to optimize public resources, ensuring that public drug plans are sustainable and have the best value for treatment as well as ensuring increased access to medications for patients.
Currently, there are about 3900 patients enrolled in Nova Scotia Pharmacare Programs who are already using a biosimilar version of a medication. Additionally, Health Canada, the regulatory agency that reviews and approves applications for biosimilars, has authorized 37 biosimilars for the Canadian market. The preliminary list of which medications will require switching to a biosimilar version can be found in the Table.
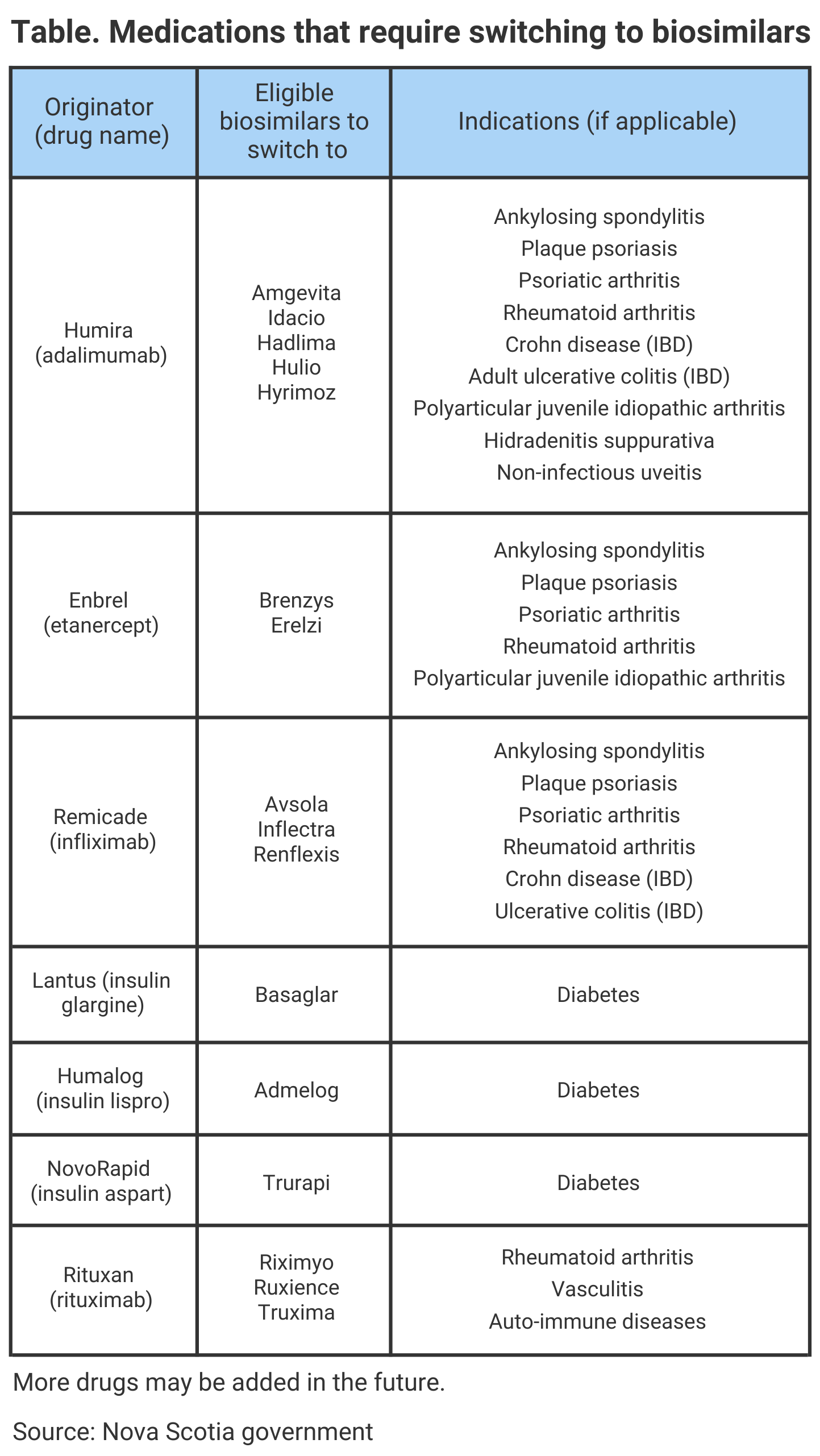
If a patient does not wish to be switched to a biosimilar, they are recommended to contact their physician to receive an exemption. A provider can apply for an exemption for clinical reasons but if it is not approved, coverage for the originator drug will end on the deadline date.
According to Biosimilars Canada, over 761,000 retail prescriptions are filled annually for biosimilars in Canada. Additionally, there are over 178 clinical trials globally that have assessed the safety of switching between reference products and biosimilars in about 21,000 patients, confirming that switching from one to the other is not associated with major efficacy, safety, or immunogenicity issues.
“Based on the experience in British Columbia, Alberta, New Brunswick, Quebec, and the Northwest Territories, where patients have already safely transitioned, we encourage the Government of Nova Scotia to work with chronic disease communities like ours to reinvest biosimilars savings in improving sustainability and adding new therapies for public drug plan beneficiaries,” said Cheryl Koehn, founder and president of Arthritis Consumer Experts, in a statement published by Nova Scotia.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
