- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
The Top Biosimilars News for the Week of July 2, 2018
Catch up on the week’s top rheumatology, gastroenterology, clinical practice, regulatory, policy, and business news from the world of biosimilars.
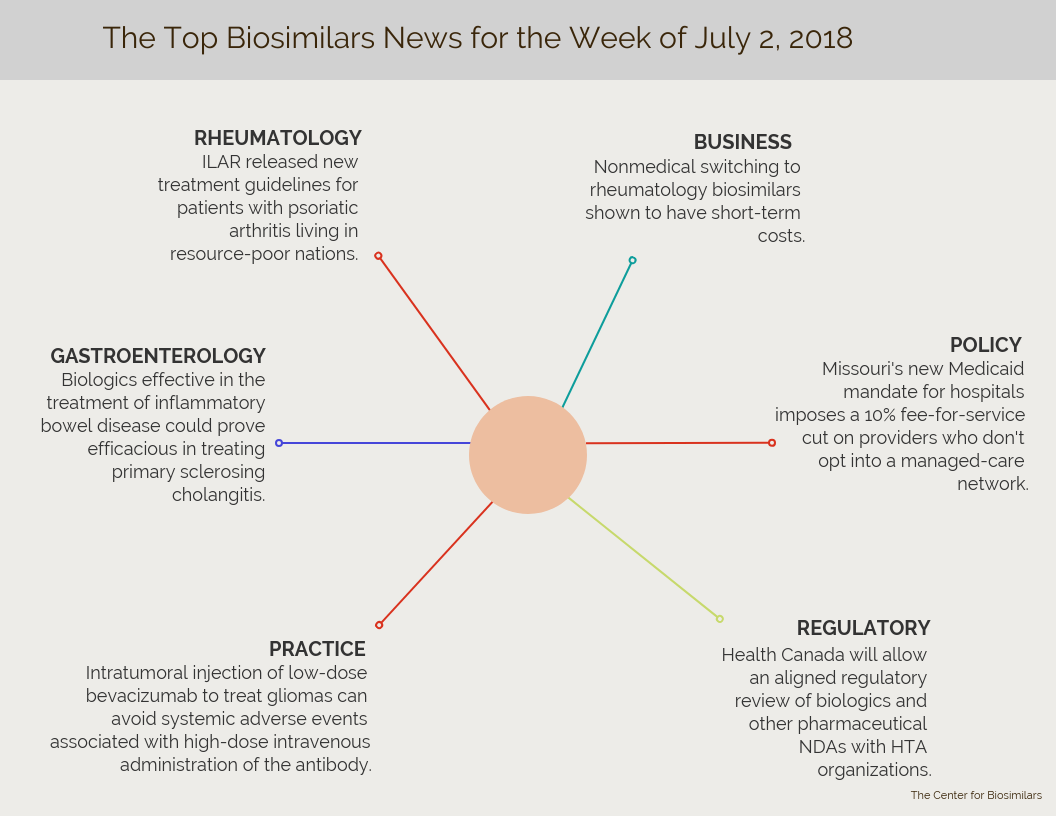
Catch up on the week’s top rheumatology, gastroenterology, clinical practice, regulatory, policy, and business news from the world of biosimilars.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
Related Content
