- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Varying Biosimilar Policies Create Uneven Market Sustainability Across Nations
An international comparative analysis found that the level of biosimilar market sustainability differs between countries, largely due to variances in biosimilar policies and years of experience managing a biosimilar market.
Although many countries have sustainable biosimilar policies to some degree, policies regarding biosimilar education and understanding need to be improved to ensure the market is sustainable long-term, according to an international study published in Frontiers in Pharmacology.
The study aimed to gain understanding about how policies can influence market success across the world and offer possible solutions to support biosimilar sustainability.
“This study proposes a set of elements that should underpin sustainable biosimilar policy development over time in a country,” wrote the authors. "At first, biosimilar policies should guarantee the safety and quality of biosimilars, healthy levels of supply and a level of cost savings. As a country gains experience with biosimilars, policies need to optimise uptake and combat any misconceptions about biosimilars."
Policies from 17 countries were assessed. The list included 3 countries from North America (Canada, Mexico, and the United States), 1 from South America (Brazil), 2 from the Asia-Pacific region (Australia and Japan), 9 from Europe (Belgium, France, Germany, Italy, the Netherlands, Norway, Spain, Switzerland, and the United Kingdom), and 2 Gulf Cooperation Council nations (Saudi Arabia and United Arab Emirates). The broad selection was to ensure that countries with different policy archetypes and different levels of economic development were represented.
Country-specific literature reviews were conducted to identify peer-reviewed articles, official government sources, and other forms of media on biosimilar policies and the results were validated by 23 international and local non-industry experts, and 2 advisory board meetings with the experts.
A framework was developed to assess policy sustainability and utilized a rating system using 5 answer categories:
- Sustainable for all stakeholders
- Minor areas for sustainability improvement
- Major areas for sustainability improvement
- Presence of unsustainable policies
- Unsustainable policy environment for majority of stakeholders
The main finding was that European countries typically had higher sustainability scores than other countries. This was unsurprising because European countries tend to have more experience with biosimilars and more developed policy frameworks. For example, the European Union and the United Kingdom have about 10 years more experience with biosimilars compared with the United States.
Policies that were considered sustainable included:
- Existing approaches to biosimilar manufacturing, research, and development
- Policies guaranteeing safe and high-quality biosimilars
- Exemption from the requirement to apply health technology assessment to biosimilars
- Initiatives counteracting biosimilar misconceptions
However, the authors noted that improvements to policies regarding biosimilar contracting approaches as well as education and understand of biosimilars can be made in all study countries.
One of the issues that arose concerned the perception of a policy’s sustainability. The authors found that some policy areas—like those regarding contracting, prescribing, dispensing, and monitoring—were rated as “sustainable for all stakeholders” in some countries but not in others (Chart).
Click to enlarge
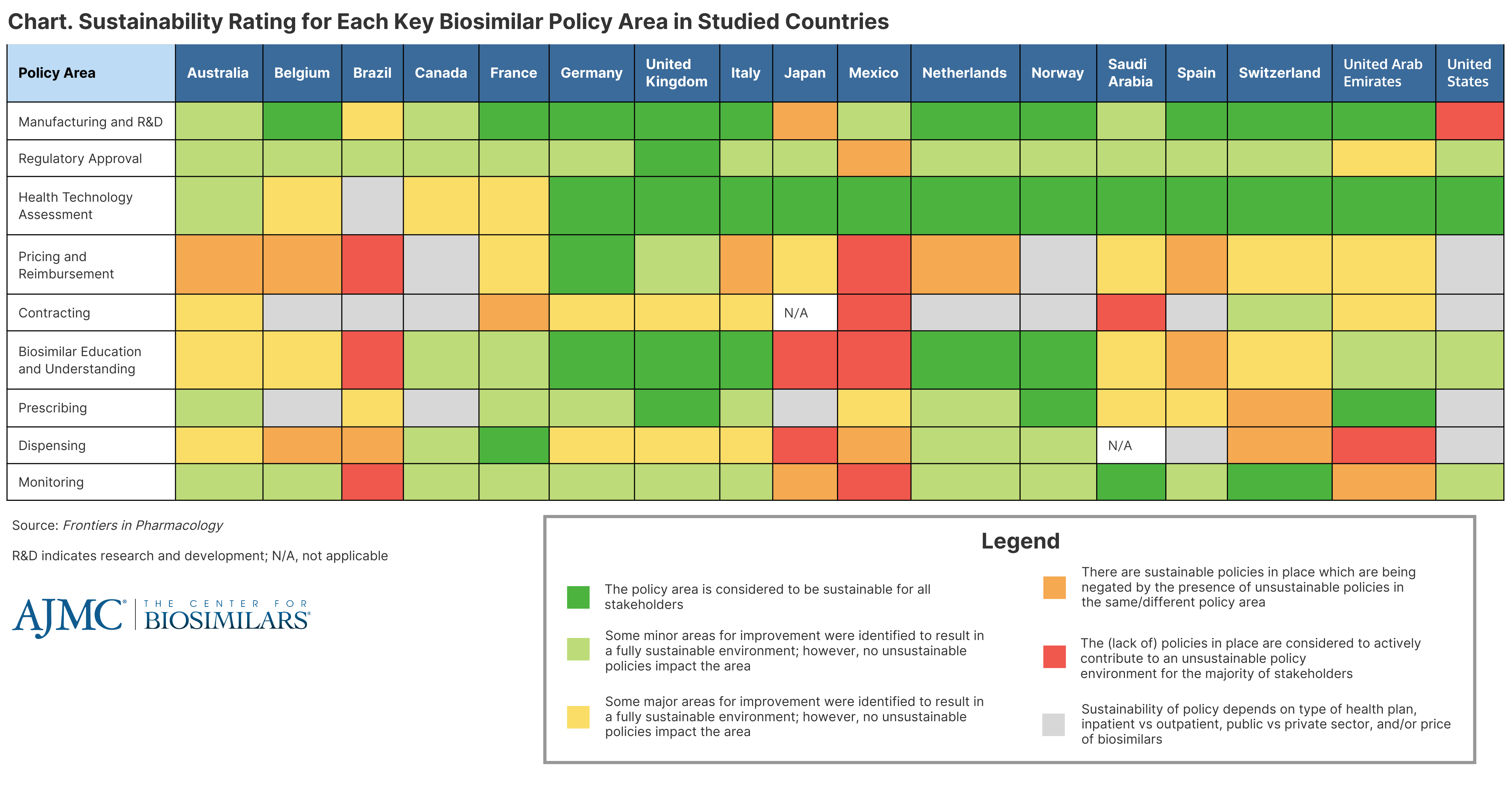
The authors had 4 main takeaways from the results on how countries should implement biosimilar policies:
- Policy introduction should be anchored in supporting industry sustainability in the short and medium term and ensure that cross-stakeholder perspectives are captured
- As a country’s biosimilar landscape matures over time and stakeholder experience increases, regulators should periodically evaluate and update policies to ensure sustainability is maintained
- Policies are less effective when implemented in a piecemeal fashion. Therefore, implementation programs should take the existing policy environment into account and leverage synergies across policy areas
- Cultivation of a sustainable global biosimilar landscape requires sharing of learning and best practices across international markets, to support accelerated development in countries with less mature biosimilar landscapes.
Reference
Alnaqbi KA, Bellanger A, Brill A, et al. An international comparative analysis and roadmap to sustainable biosimilar markets. Front Pharmacol. Published online August 24, 2023. doi:10.3389/fphar.2023.1188368
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
