- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Abrilada Approved as Second Interchangeable Humira Biosimilar
Pfizer’s Abrilada (adalimumab-afzb) became the second biosimilar referencing Humira (adalimumab) to receive an interchangeable designation. The new label is intended to expand patient access to biosimilars.
The FDA granted interchangeability to Abrilada (adalimumab-afzb), the second adalimumab biosimilar to receive the designation. It is also the fifth biosimilar overall to be deemed interchangeable.
Interchangeability designations allow for a biosimilar to be substituted for a reference product at the pharmacy level without having to obtain permission from a physician. The label is intended to reduce wait times for patients to get their medications and to increase accessibility to biosimilars.
Abrilada was originally approved by the FDA in November 2019. Of the 9 FDA-approved adalimumab biosimilars, Abrilada is the only one left to launch on the US market. In January, Amjevita (adalimumab-atto) launched. In July, 7 more entered the market, including Cyltezo (adalimumab-adbm), the only other adalimumab biosimilar with an interchangeability label.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” said Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, in Pfizer’s statement.
The FDA based its decision on data from the phase 3 REFLECTIONS B538-12 study, which demonstrated the safety and efficacy of switching between Humira and Abrilada in patients with moderate to severe rheumatoid arthritis (RA). Patients were switched between the 2 products multiple times and all patients were taking adalimumab in combination with methotrexate.
Pfizer said that Abrilada will be available later this year and will launch at 2 price points, similar to how Amjevita and several other adalimumab products launched (Table). Abrilada will be offered as a low-concentration, citrate-free formulation.
Adalimumab WAC Trends Q3 2023
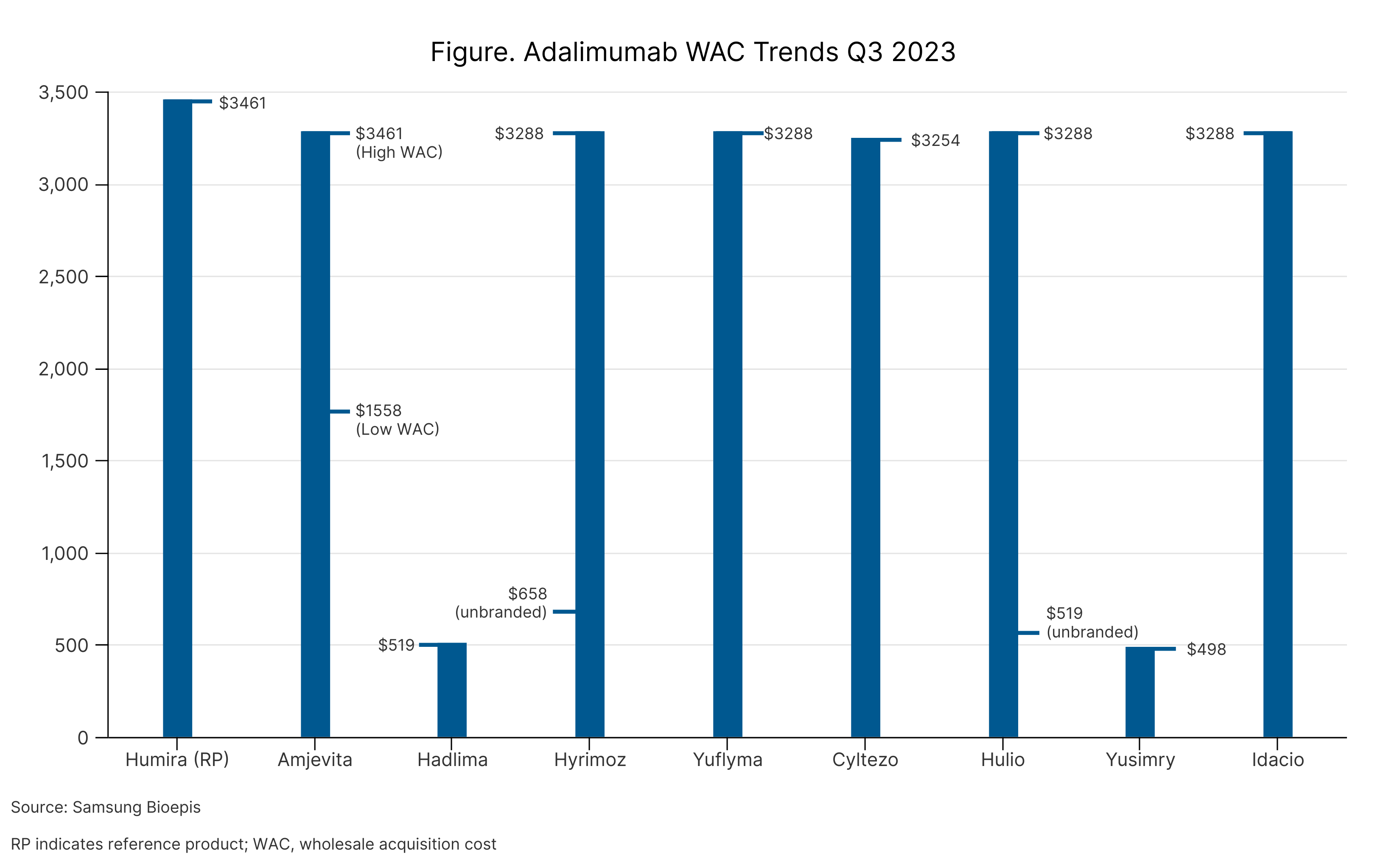
Adalimumab products are tumor necrosis factor-⍺ inhibitors used to treat several rheumatic and gastroenterology conditions, including RA, psoriatic arthritis, juvenile idiopathic arthritis, Crohn disease, ulcerative colitis, ankylosing spondylitis, and plaque psoriasis.
“Abrilada was developed with patients in mind, and having an interchangeable designation is a key step toward potentially increasing their access to this important treatment option,” said Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business, Pfizer. “With 15 years of global in market experience in biosimilars and an industry-leading seven marketed biosimilar products in the U.S., Pfizer continues to expand options for patient care by introducing Abrilada.”
In addition to Abrilada and Cyltezo, 2 insulin glargine biosimilars (Rezvoglar and Semglee) and 1 ranibizumab product (Cimerli) have interchangeability designations. Alvotech, Celltrion, and Organon/Samsung Bioepis are also working to obtain interchangeability for their respective adalimumab biosimilars.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
