- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Alvotech Reports Positive Switching Results for AVT02 Adalimumab Candidate
The findings support Alvotech's bid for interchangeable status for the high-concentration, citrate-free adalimumab biosimilar candidate.
The Icelandic biosimilar developer Alvotech reported positive top-line results of a study of patients who switched between the company’s proposed high-concentration (100 mg/mL), citrate-free adalimumab biosimilar (AVT02) and the originator product (Humira). Top-line clinical results are the audited, comprehensive findings from a trial.
The study could potentially help Alvotech gain US “interchangeable status” for AVT02. An interchangeable designation means pharmacists can substitute a biosimilar for the originator drug at the pharmacy counter without the intervention of a physician. Such a designation could give the product an edge over other adalimumab biosimilars—of which 6 have been approved but none marketed.
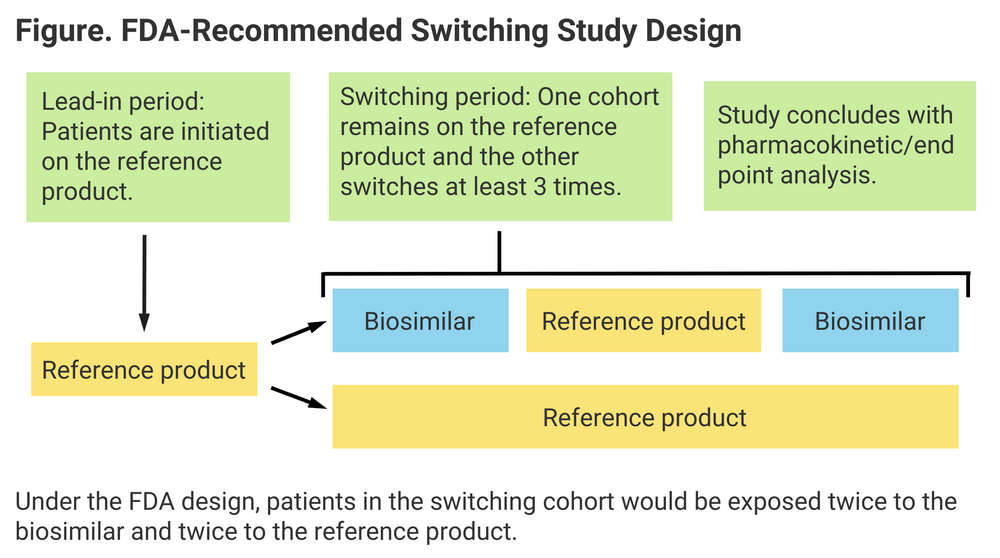
For the interchangeable designation, switching studies are required by the FDA to demonstrate that similar patient outcomes can be expected whether patients switch between originator and biosimilar once or more than once. Just 1 biosimilar currently on the US market has an interchangeable designation: Semglee (insulin glargine).
Alvotech reported that no significant differences were observed in clinical efficacy, safety, or immunogenicity between patients in the switching cohort and those who remained on the reference product throughout—the control group. The product would be aimed at the lucrative Humira franchise, which recorded US sales of $16.1 billion in 2020, up 8.4%, partly on the strength of price increases that exceeded the rate of drug inflation.
“Completing this key milestone for Alvotech is an extremely rewarding and a positive step toward providing patients an affordable and accessible alternative to the high-concentration version of Humira,” said Mark Levick, CEO of Alvotech, in a statement.
For the switching study (AVT02-GL-302; NCT04453137), 568 patients were enrolled from roughly 30 clinical centers in the European Union. All patients were initiated on reference product during a lead-in period of 12 weeks, and eligible patients were then randomized to either the switching arm or the control group. The switching cohort received alternate doses of AVT02 and Humira for an additional 16 weeks.
The graphic accompanying this story shows the FDA’s recommended switching study model (Figure).
Boehringer Ingelheim also is seeking US interchangeable status for an adalimumab biosimilar (Cyltezo; BI 695501). This product is a lower-concentration formulation (40 mg/0.8 mL) than AVT02.
In February 2021, Celltrion Healthcare obtained EU marketing authorization for a high-concentration, citrate-free adalimumab biosimilar (Yuflyma). This was the first high-concentration adalimumab biosimilar to reach market.
Alvotech remains engaged in litigation over AVT02 with AbbVie, maker of Humira, which has alleged patent infringement.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.