
- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Analysis: G-CSF Biosimilars Could Reduce Costs While Improving FN-Related Outcomes
Wider use of granulocyte colony-stimulating factor (G-CSF) biosimilars is recommended for patients at risk of febrile neutropenia (FN).
Febrile neutropenia (FN) in patients undergoing chemotherapy has long been counteracted with granulocyte colony-stimulating factor (G-CSF) drugs. Models of G-CSF biosimilar use in this setting suggest not only significant savings but also potential for expanded access.
FN is defined as fever and possible infection in combination with a low white blood cell count (neutrophil granulocytes). Guidelines limit the use of G-CSFs as prophylaxis for FN, although during the pandemic in the United States, oncologists broadened use of these agents to reduce hospitalization risk and COVID-19 infection. Savings have been associated with this expanded use. Further, in the Oncology Care Model (OCM), where practices are tasked with reducing costs of care while maintaining quality of care, CMS documented a 50% increase in use of biosimilar pegfilgrastim from 2014 to 2018.
G-CSF drugs include filgrastim (originator, Neupogen) and the pegylated, longer-acting form pegfilgrastim (originator, Neulasta), both of which are available as biosimilars. The pegfilgrastim reference product is available as a prefilled syringe (PFS) or an onbody injector (OBI) that automatically delivers a single dose of pegfilgrastim. Biosimilar G-CSF pegfilgrastim and filgrastim products addressed in the current study were Ziextenzo and Zarxio, respectively.
G-CSF for Patients at High Risk of FN
According to the authors of a recent study published in JCO Oncology Practice, clinical guidelines clearly indicate G-CSF for patients at high risk of FN; however, the recommendations for patients at intermediate risk “are less clear.” These call for clinical judgment based on the individual patient’s situation. The authors cited recent evidence suggesting that routine, primary prophylaxis with G-CSF in patients at intermediate risk “may lead to reductions in health care utilization and costs.” A major reason for lower rates of prophylaxis with G-CSF products, they said, could be the historically high costs of reference G-CSF drugs.
The investigators conducted their simulation with the OCM in mind. OCM practices receive performance-based payments if they meet their quality and cost-savings goals. The authors hypothesized that a reduction in drug-related costs through the use of biosimilars and in FN-related hospitalizations through expanded access of intermediate-risk patients to G-CSF prophylaxis would help practices meet these goals.
Their economic analysis simulated cost savings in a US practice associated with (1) switching from originator G-CSF products to biosimilars and (2) switching to biosimilars and expanding access to 10% more intermediate-risk patients. They conducted a 1-year economic analysis of real-world drug costs and health care resource utilization (HCRU) in 500 patients in a hypothetical US oncology practice.
The simulated cohort of patients had a “heterogeneous mix” of nonmyeloid cancers and received 6 cycles of chemotherapy over a 1-year period. Patients were classified as being at high risk of developing FN (greater than 20%), intermediate risk (10%-20%), or low risk (< 10%). The proportions of patients receiving G-CSF prophylaxis were based on previously published real-world evidence: 27 of 45 (59%) patients at high risk, 70 of 240 (29%) patients at intermediate risk, and 24 of 215 (11%) patients at low risk were treated with G-CSF. Of these 121 patients treated with G-CSF, the model estimated 6 would experience an FN event.
Drug costs were derived from current wholesale acquisition costs and administration costs from physician fee schedules, published by the Centers for Medicare and Medicaid Services in April 2020. Nursing labor costs were estimated using national average nurse wages in 2020.
In Model 1, the authors simulated the cost savings associated with use of biosimilars compared with originator G-CSF medications. FN-related costs were not considered and access to G-CSF prophylaxis was not expanded. The cost savings ranged from $537,000 for use of a biosimilar in place of filgrastim to $1.68 million for a biosimilar in place of pegfilgrastim OBI. They noted that the cost of pegfilgrastim OBI is the same as pegfilgrastim PFS; however, administration costs are higher, accounting for the greater savings when switching from pegfilgrastim OBI to biosimilar pegfilgrastim (Table 1).
Click to enlarge.
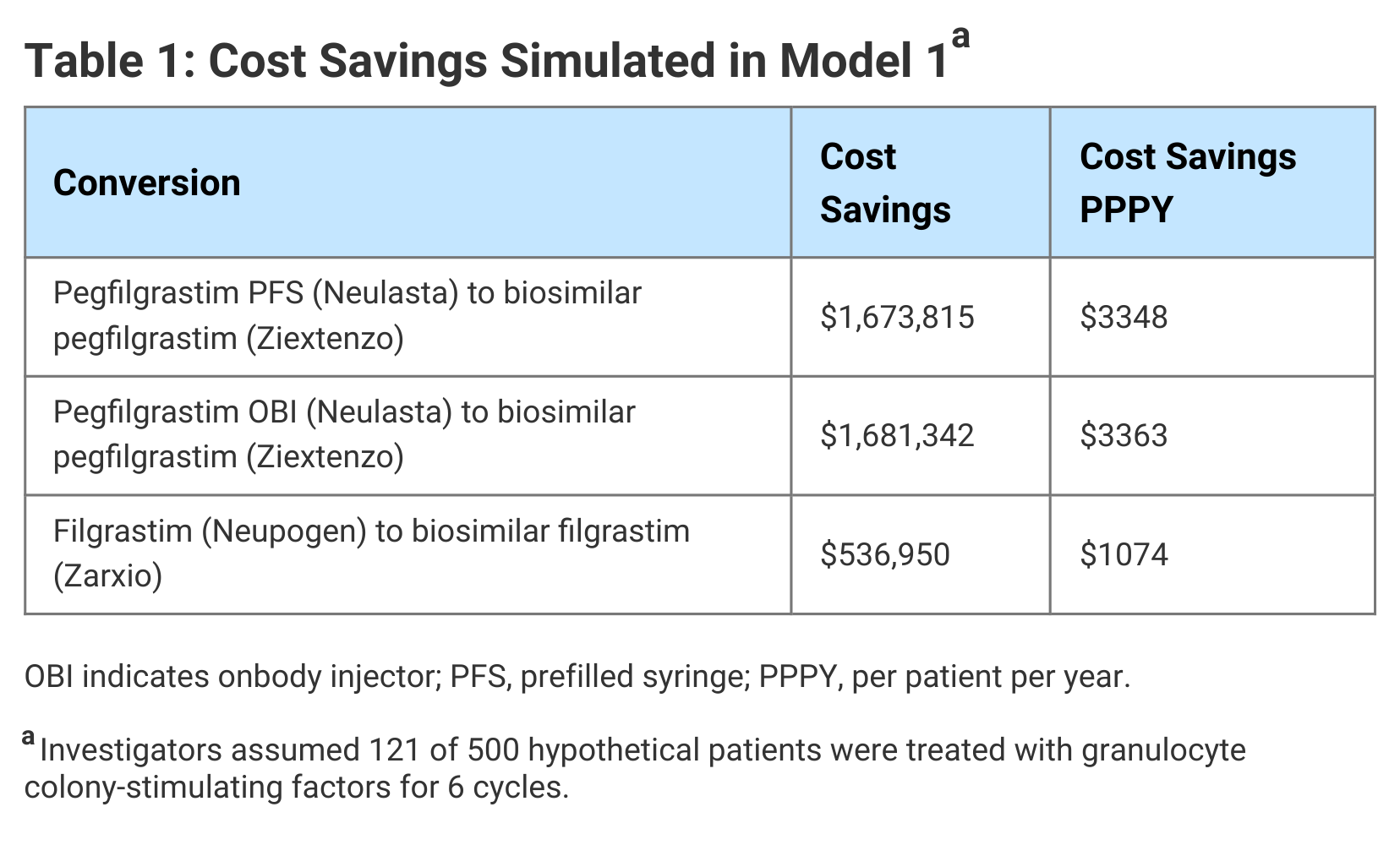
In Model 2, in addition to the conversion to biosimilars, hypothetical cost savings were reinvested to provide 10% more patients (n = 24) at intermediate risk of FN with biosimilar G-CSF. The number of high- and low-risk patients receiving G-CSF prophylaxis was unchanged. In this model, it was estimated 7 patients who received G-CSF would experience FN events.
Total cost savings in the expanded access scenario simulated in Model 2 ranged from approximately $25,000 for a change from originator to biosimilar short-acting filgrastim to $1.19 million for a change from pegfilgrastim OBI to a biosimilar. In Model 2, cost savings in HCRU were realized, because the expanded access to G-CSF allowed for reduced FN-related costs (Table 2).
Click to enlarge.
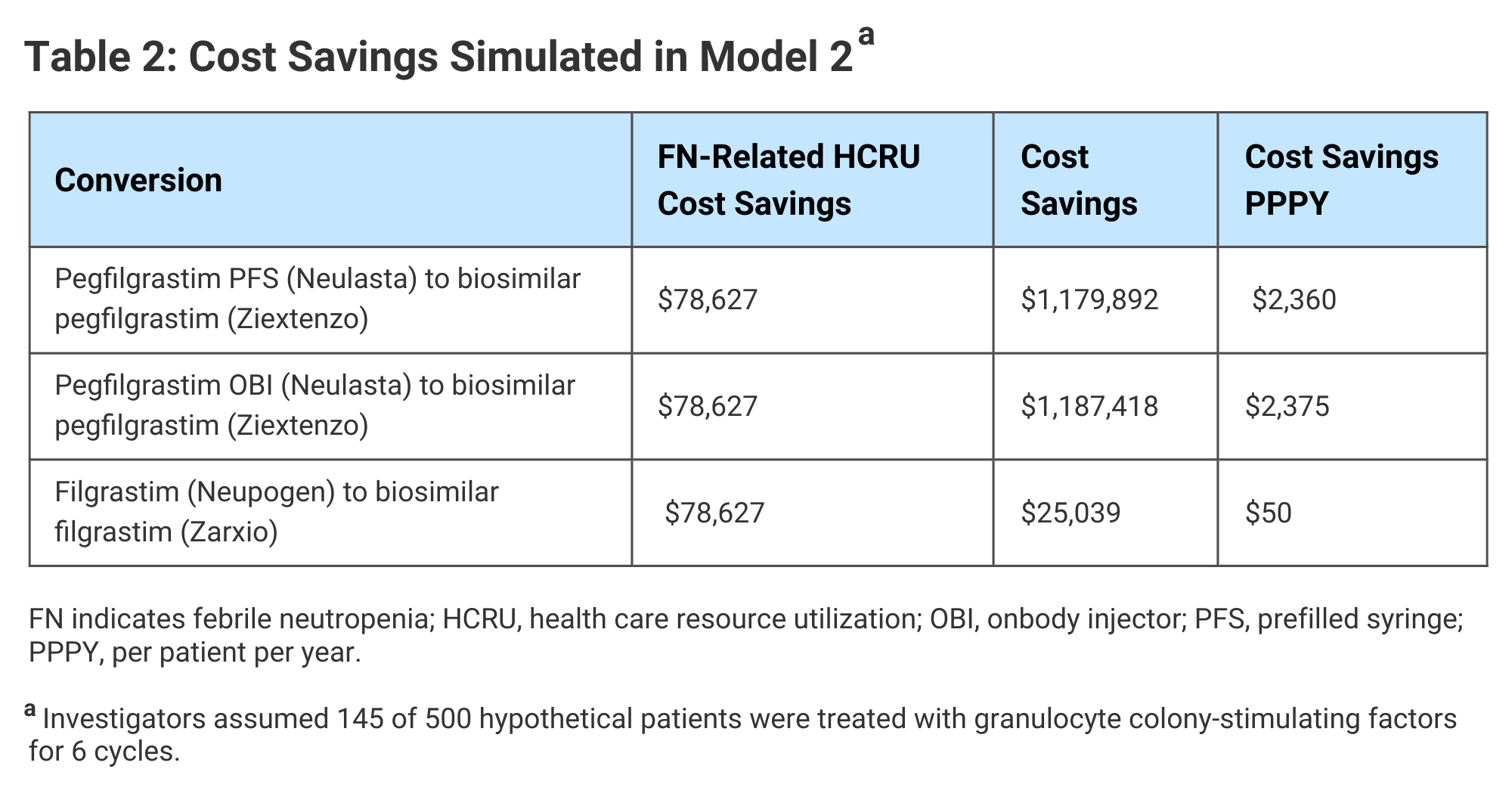
The investigators said their analysis demonstrated both “substantial” cost savings and improved clinical outcomes from the use of biosimilar G-CSF drugs in patients undergoing myelosuppressive chemotherapy and that these findings are especially relevant to oncology practices striving to meet OCM goals.
“Conversion to biosimilar G-CSFs would allow more patients at intermediate risk of FN to be treated with prophylactic biosimilar G-CSF, with fewer patients experiencing FN events and still generating savings to the practices and health care system,” they said.
Reference
Wang W, Li E, Campbell K, McBride A, D'Amato S. Economic analysis on adoption of biosimilar granulocyte colony-stimulating factors in patients with nonmyeloid cancer at risk of febrile neutropenia within the Oncology Care Model framework. JCO Oncol Pract. Published online May 7, 2021. doi:10.1200/OP.20.00994
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
259 Prospect Plains Rd, Bldg H,
Cranbury, NJ 08512
All rights reserved.