- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
BioRationality—A Dr. Sarfaraz Niazi Column: Lessons From 2022 Biosimilar Events
Sarfaraz K. Niazi, PhD, summarizes some of the lessons that the entire biosimilar industry can take away from 2022, including what new legislation and policy changes could mean for future biosimilar development.
A year-end is a way to define a time boundary that allows me to summarize a few events of relevance to biosimilars and offer brief perspective comments on how to benefit from the experience of the year 2022, both for developers and regulatory agencies.
- FDA approves first biosimilar without clinical efficacy trials (Releuko, filgrastim biosimilar)
- While the FDA has stated that a clinical efficacy (in-patient) study may not be required, this first approval establishes a precedent. As a result, developers can expect to secure sufficient data from clinical pharmacology studies alone for products with pharmacodynamic endpoints and soon for all products.
- FDA approves first interchangeable biosimilar without switching study (Cimerli, ranibizumab biosimilar)
- This approval establishes the validity of clinical pharmacology testing and offers opportunities to secure interchangeability using in silico studies.
- The Biologics Price Competition and Innovation Act (BPCIA) amended to remove "animal toxicology" and replaced it with "nonclinical."
- The FDA Modernization Act 2.0 also included a bill to reduce animal testing for both 505B2 and 351k submissions; given that the animal testing of biosimilars has been criticized widely, this amendment to the BPCIA will mean that developers should no longer plan these studies.
- Senator Mike Lee’s (R-Utah) bill to stop the FDA from giving interchangeable status to biosimilars.
- Efforts are underway to block the FDA from awarding interchangeable status as it creates more doubts about the safety of biosimilars, and such testing can always succeed. This Bill is expected to pass in 2023. The author assisted in the writing of the bill.
- Adalimumab, pegfilgrastim, and infliximab biosimilars account for half of all biosimilars approved. The total number of molecules increased to 11. (Figure 1)
- Developers are betting on molecules that have already been approved to avoid litigation and development risks. However, there is an enormous opportunity to move beyond these 11 molecules.
Click to enlarge.
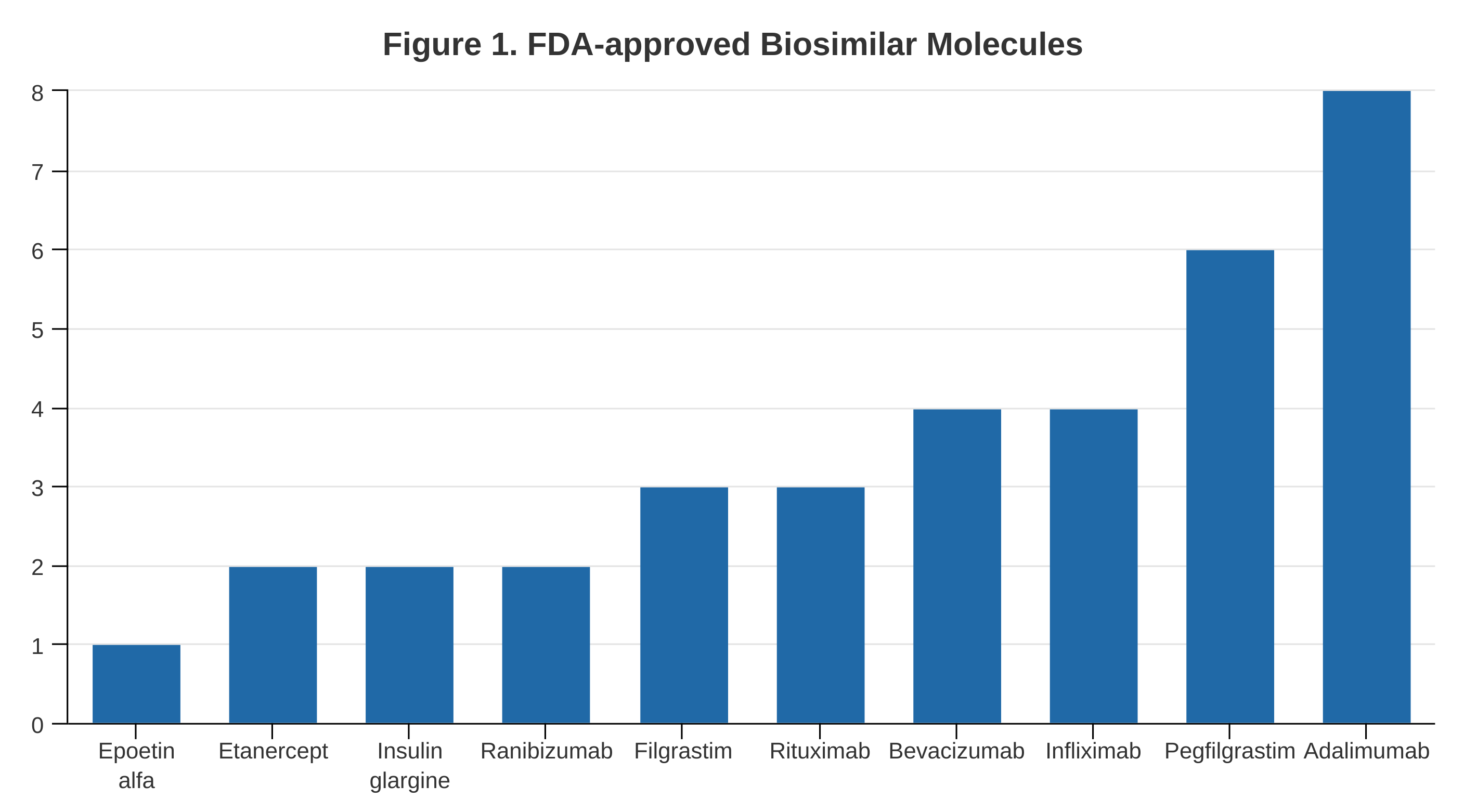
- Four new patent litigation cases were filed; 30 were resolved from the past. However, no biosimilar was prevented from entering the market.
- The fact that no biosimilar was blocked from entering the market tells that the "patent dance" is merely a hurdle that should be removed by a legislative change that will penalize the reference product companies if their patent claims are not proven.
- A cyclical trend of a rising number of approvals is likely to return. (Figure 2)
Click to enlarge.
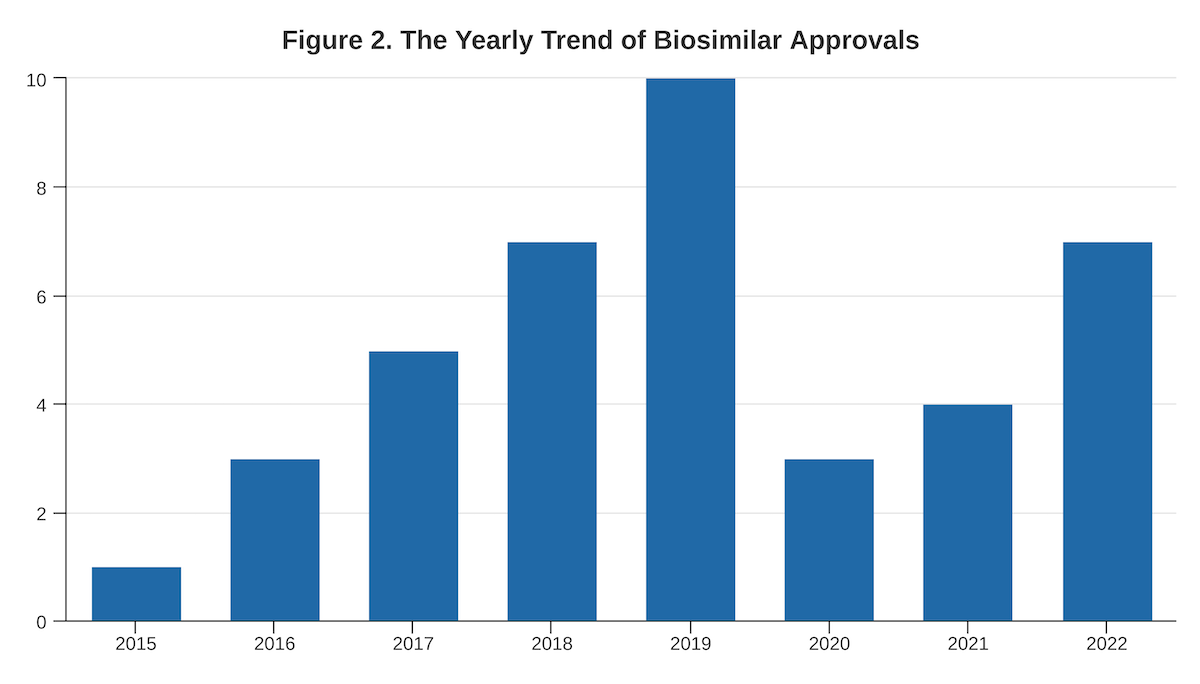
- A trend peaking in 2019 is rising again following the previous pattern; more biosimilars are expected to be approved in 2023 than in 2022.
- FDA launched a new Biosimilar Regulatory Science Program to encourage applications of new sciences to establish biosimilarity.
- New technologies based on artificial intelligence can be used to establish biosimilarity based on the AlphaFold2 3D structure prediction based on the basis that if the primary sequence of the biosimilar is identical, then its 3D structure and thus efficacy and immunogenicity should be the same.[1], [2]
- New technologies based on artificial intelligence can be used to establish biosimilarity based on the AlphaFold2 3D structure prediction based on the basis that if the primary sequence of the biosimilar is identical, then its 3D structure and thus efficacy and immunogenicity should be the same.[1], [2]
References
[1]. Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583-589. doi:1038/s41586-021-03819-2
[2]. Niazi SK. Molecular Biosimilarity-An AI-Driven Paradigm Shift. Int J Mol Sci. 2022;23(18):10690. doi:10.3390/ijms231810690.
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
