- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
BioRationality: FDA Final Guidance on Continuous Manufacturing—A Boon for Biosimilars
The misconceptions about disposable bioreactors' lifecycle are gone; the era of hard-piped, giant, stainless-steel tanks for biosimilar development is history now, according to Sarfaraz K. Niazi, PhD, in his latest column.
Source: DALLE-Open AI

The days of giant bioreactors and billions of dollars of investment are gone. Now a small bioreactor, no more than 1,000 L, can produce a commercial supply of biologics in a continuous mode, a quest that has been in the works for several years.[1]
In March 2023, the FDA sealed it in a detailed final guidance, addressing all validation and compliance issues, and details of lifecycle management. It applies to both chemical and biological products. Now all new biologics and biosimilars can be produced with the least capital expenditure and operating expenditure. This should encourage smaller entities to enter the biosimilars business.
In addition, it should be made clear that the Biologics Price Competition and Innovation Act restricts how a product is approved. For example, contiguous manufacturing applies to perfusion systems where proteins are secreted. In contrast, both eukaryotic and prokaryotic cells secrete, and continuous manufacturing is most suitable for eukaryotic systems that allow post-translational modification, such as significant dose antibodies.
A model of the steps using bioreactors for pharmaceutical development
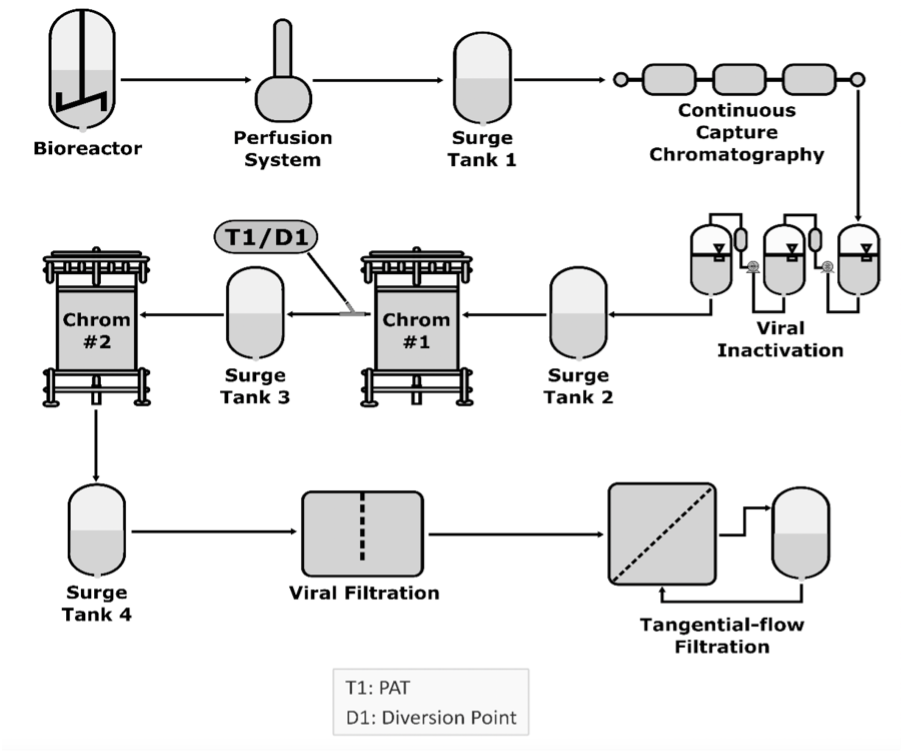
Continuous manufacturing applies to any unit operation, such as a bioreactor, capture chromatography, virus filtration, virus inactivation, buffer exchange, and concentration through tangential flow chromatography. Each unit operation is integrated with adjacent unit operations, a surge line, or a tank connecting unit operations.
Using a surge line or tank allows continuous operations to accommodate differences in mass flow rates or process dynamics. It involves continuously feeding input materials into, transforming in-process materials within, and simultaneously removing output materials from a manufacturing process in an integrated system with 2 or more unit operations, regardless of their nature. Integrating the fill and finish unit operations is not too far, further reducing the cost. The idea of an integrated modular system from cell culture to vials and syringes is forthcoming; such an example has already been applied to messenger RNA production.
The batch testing cost is significantly reduced as continuous monitoring systems remove many testing requirements. Other high-cost components of at-scale process performance qualification lots are decreased substantially, and so is the time to file a biologics license application (BLA) since a single run can provide process and product validation. For analytical assessment in the development of biosimilars, samples drawn over the cycle period can be used to compare critical quality attributes, another significant saving to developers. I have filed my query in the comment section of the FDA portal.
The FDA has also listed additions to the Common Technical Document for the Registration of Pharmaceuticals for Human Use specific to continuous manufacturing BLA submissions, making it clear to developers what they need to submit.
The critical elements of the guideline include:
- The batch size can be fixed, or a defined range must be justified. If going beyond the defined range, a post-approval change will be required.
- The batch size produced by continuous manufacturing is defined as the quantity of output material, input material, and run time (minimum or maximum) at a defined mass flow rate. In continuous manufacturing processes, a single thaw of one or multiple vials from the same cell bank may result in single or various harvests.
- Changing the manufacturing mode from batch to continuous requires a control strategy to establish product comparability and assess the need for additional bioequivalence, nonclinical and clinical studies, and stability data.
- Drug substance in-line release tests for pH, osmolality, and protein concentration and online release tests for purity, charge heterogeneity, aggregation, and low molecular weight entities can be performed at specific points in the drug substance manufacturing process shown to be critical for control of the product quality attributes.
- Extended cell culture period validation and ongoing drug substance extraction from harvested cell culture material are exclusive to continuous manufacturing. This calls for proof that every piece of cell culture material utilized to produce a certain medicinal ingredient batch is acceptable. In addition, new technologies for real-time decision-making, such as rapid testing for adventitious agents, may mitigate the impact of contamination events through early detection and appropriate responses during continuous operation.
- The release test performed on the output material may be a surrogate for a traditional release testing method. For example, conventional offline testing for product release is necessary for quality attributes for which analytical technologies are unavailable for online or in-line measurements (eg, potency). Likewise, conventional tests for monitoring and control (eg, microbiological analytical methods and other tests that require long processing times) might also be needed.
- While continuous manufacturing bioreactors operate longer than bioreactors for batch manufacturing, the in vitro cell age limit for production cells remains the same. Therefore, previously established limits of in vitro cell age for a bioreactor operating in a batch mode run may not apply to a bioreactor operating in a continuous mode under different culture conditions. The in vitro cell age limit used for production should be based on information obtained from production cells developed to the recommended in vitro cell age or beyond under pilot-plant size or commercial scale circumstances.
- Although the drug substance is not isolated, the origin and fate of potential impurities (e.g., related substances, residual solvents, catalysts), the robustness of impurity clearance, and impurity carryover from the drug substance into the drug product should be provided in the dossier. In addition, the control of impurities formation and clearance should be integrated into the overall control strategy.
- The impurities in the drug product specification may differ from those in the drug substance specification.
- Drug substance stability data to define a retest period is not applicable because the drug substance is not isolated and stored in an integrated process.
I do not see any reason why a biosimilar developer would not choose continuous manufacturing over batch manufacturing. Also, the approved biosimilars can be switched over to secure better profit margins as the competition heats up.
Source: DALLE-Open AI

However, to reduce the time and cost of switchover, careful planning is required to minimize the switching cost. Setting up new manufacturing is more straightforward, and maximizing single-use technologies is recommended. The misconceptions about disposable bioreactors' lifecycle are gone. The era of hard-piped giant stainless-steel tanks, a darling of Big Pharma, is history now.
Reference
[1] National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Chemical Sciences and Technology. Continuous Manufacturing for the Modernization of Pharmaceutical Production: Proceedings of a Workshop. Washington (DC): National Academies Press (US); January 30, 2019. Accessed April 12, 2023. https://pubmed.ncbi.nlm.nih.gov/30994997/
Newsletter
Where clinical, regulatory, and economic perspectives converge—sign up for Center for Biosimilars® emails to get expert insights on emerging treatment paradigms, biosimilar policy, and real-world outcomes that shape patient care.
