- Bone Health
- Immunology
- Hematology
- Respiratory
- Dermatology
- Diabetes
- Gastroenterology
- Neurology
- Oncology
- Ophthalmology
- Rare Disease
- Rheumatology
Biosimilar Candidate CT-P43 Shows Biosimilarity With Ustekinumab Originator in Plaque Psoriasis
Similar efficacy, safety, pharmacokinetics, and immunogenicity were found when comparing Stelara, originator ustekinumab, and CT-P43, an ustekinumab biosimilar candidate, in patients with moderate to severe plaque psoriasis.
Dermatologist examining skin | Image Credit: fusssergei - stock.adobe.com
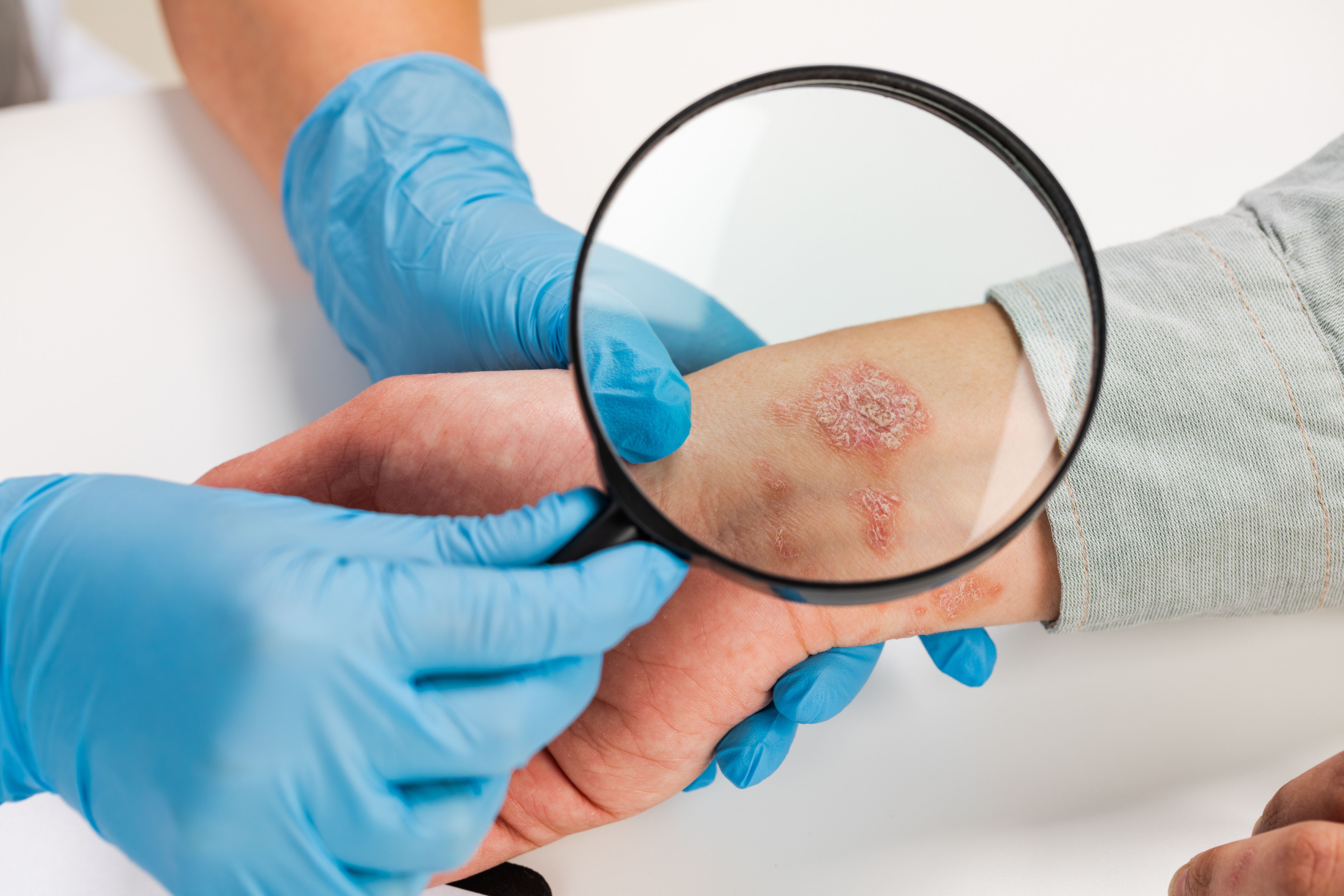
A randomized, double-blind study found comparable efficacy, safety, pharmacokinetics (PKs), and immunogenicity between Stelara, the ustekinumab originator, and the biosimilar candidate, CT-P43 for patients with moderate to severe plaque psoriasis.
Patients varied among 34 different centers across Estonia, Poland, Ukraine, and the Republic of Korea to participate in 2 treatment periods ranging from January 2021 to November 2021. Treatment period I randomized patients to receive either the originator ustekinumab or CT-P43 from baseline to week 16. Treatment period II rerandomized patients to either continue receiving the originator, continue CT-P43, or switch from the originator to CT-P43 from week 16 to week 40. Eligible patients were 18 to 80 years old with more male patients than female in both the originator (male, n = 173, F: 80) and CT-P43 (M: 161, F: 95).
A total of 509 patients participated in treatment period I with 253 receiving the originator and 256 receiving CT-P43. Drug administration took place at week 0 and week 4 where patients less than or about 100 kg received 45 mg doses while patients more than 100 kg received 90 mg doses.
Treatment period II randomized patients to continue the originator, switch to CT-P43, or continue CT-P43 from week 16 to week 40 (n = 125, 124, 253, respectively). Drug administration took place at weeks 16, 28, and 40 with doses adjusted at week 16 pre-dose to reflect any weight changes. Near the end of the study, 3 patients discontinued CT-P43 in the continued group (n = 250) and 2 patients discontinued treatment in the originator group (n = 123) while the switched group did not have discontinuations.
Efficacy results were comparable among treatment periods in improved Psoriasis Area and Severity Index (PASI) scores. At baseline, the average PASI score was 21.51 in the CT-P43 group and 20.88 in the ustekinumab group. At week 12, the proportions of patients who achieved PASI scores of 50/75/90/100% were 86.05 for CT-P43 and 83.99 for ustekinumab. By week 28, the proportions of patients who experienced improvements in their PASI scores were 92.55% in the continued ustekinumab group, 95.07% in the continued CT-P43 group, and 92.86% in the switched groups.
Efficacy endpoints included patients’ Dermatology Life Quality Index (DLQI) score with patients in the CT-P43 group (DLQI, 13.2) and the originator group (DLQI, 11.9) at baseline. By week 12, the mean changes from baseline in DLQI score were –9.7 and –8.5 for CT-P43 and the originator groups, respectively. At week 28, the continued CT-P43 group, continued originator group, and the switched to CT-P43 group had mean DLQI score changes of –10.9, –8.8, and –9.4, respectively.
Safety was evaluated based on treatment-emergent adverse events (TEAEs) where 18 patients in CT-P43 and 15 in the originator groups experienced at least 1, none leading to discontinuation. Treatment-emergent serious adverse events (TESAE) were present in 4 patients in each group. The continued CT-P43 group, continued originator, and switched to CT-P43 group had 4, 5, and 6 patients, respectively, with a drug experience related to TEAE but no TESAEs. The majority of TEAEs were grade 1-2 in intensity. Baseline injection-site reactions (ISRs) 3 patients in the biosimilar group and 2 in the originator group, but 0 patients in the continued groups experienced them. Those who switched to CT-P43 had an ISR in 2 patients.
Three patients discontinued the study drug in the biosimilar group and 4 discontinued in the originator ustekinumab group.
PKs were consistent with serum ustekinumab concentrations at all timepoints measured. Treatment groups and dose were generally similar, including after transitioning from originator to biosimilar candidate or vice versa.
Regarding immunogenicity, patients in the CT-P43 group had lower results of positive antidrug antibodies (ADAs) compared with the originator in treatment period I. No major change in ADA frequency occurred in period II. By week 28, ADA positive patients included 26 in the CT-P43 group, 21 in the continued reference product group, and 22 who switched to CT-P43. Treatment period I had slightly lower ADA-positive serum concentrations vs ADA-negative subgroups among both treatment II groups. There were no correlations in treatment period I between ADA positivity and TEAEs, TESAEs, ISRs, or hypersensitivity reactions.
Limitations included singular focus on the switch rather than the absence of multiple switch combinations throughout the study design. Patients and other investigators remained blinded but staff administering drugs could not be blinded. The study also took place during the COVID-19 pandemic and excluded patients with protocol deviations.
Reference
Papp KA, Lebwohl MG, Thaçi D, et al. Efficacy and safety of candidate biosimilar CT-P43 versus originator ustekinumab in moderate to severe plaque psoriasis: 28-week results of a randomised, active-controlled, double-blind, phase III study. BioDrugs. 2024;38(1):121-131. doi:10.1007/s40259-023-00630-5
Escaping the Void: All Things Biosimilars With Craig & G
May 4th 2025To close out the Festival of Biologics, Craig Burton and Giuseppe Randazzo from the Association for Accessible Medicines and the Biosimilars Council tackle the current biosimilar landscape and how the industry can emerge from the "biosimilar void."
Biosimilars Gastroenterology Roundup for November 2024—Podcast Edition
December 1st 2024On this episode of Not So Different, we discuss market changes in the adalimumab space; calls for PBM transparency and biosimilar access reforms grew; new data for biosimilars in gastroenterology conditions; and all the takeaways from this year's Global Biosimilars Week.
